Crude oil is the raw material from which alkanes are extracted via the process of fractional distillation. There is nothing more to this than you learnt at GCSE but you need to be able to briefly describe the process (and be clear that we are simply separating out molecules – intermolecular bonds are being broken when the oil is vaporised, not covalent bonds).
If you need a refresher, check this out:
Each fraction is a mixture of molecules with similar boiling points and numbers of carbon atoms. The gasoline fraction (bpt range 25-75°C; 5-7 carbon atoms) is used to make petrol but it has to further refined before it is fit for use. The other problem is that most crude oil is rich in high boiling fractions such as gas oil and residue, but not the more useful lighter fractions.
The length of the hydrocarbon chain determines how each fraction is further refined and is summarised in the table below:
| Isomerisation | Reforming | Steam cracking | Catalytic cracking | |
| Feedstock | C4 – C6 alkanes | C6 – C10 alkanes | C6 – C16 alkanes | C14 – C20 alkanes |
| Conditions | Pt / Al2O3 catalyst, 150°C | Pt / Al2O3 catalyst, 500°CHydrogen is added to the mix to reduce coking of the catalyst surface | No catalyst, 900°CSteam is added to the mix to reduce coking | Zeolite catalyst, 500°C |
| Products | Produces branched chain isomers | Produces alicyclic and aromatic molecules | Produces shorter straight chain alkanes and alkenes | Produces branched chain alkanes and alkenes |
Cracking
Cracking breaks down long chain alkanes into short chain and branched chain alkanes, cycloalkanes and alkenes. The mixture of products is then fractionally distilled to separate them out from each other.
The basics of cracking are covered in the video below:
Industrially, catalytic cracking produces a greater yield of high octane gasoline and alkenes than thermal cracking and so is the preferred process. Heavy gas oil is vaporised at high temperatures and mixed with a fluidised powdered zeolite catalyst in the riser column. The long chain molecules are cracked into shorter chain alkanes (straight chain and branched), some cyclic alkanes and alkenes.

The cracking reactions produce coke (carbon) which coats the catalyst and reduces its efficiency. This spent catalyst flows through steam to remove hydrocarbon vapours and is then regenerated by burning off the coke deposits in air. The heat produced in these reactions is used to both vaporise the heavy gas oil feedstock and provide energy for the cracking reactions which are endothermic, lowering energy costs overall.

The catalyst is a type Y zeolite. Zeolites are either naturally occurring or synthetic minerals with a crystal structure. They contain an extensive network of interlocking pores and channels which give the zeolite a very large surface area for catalysing reactions and they are also used as molecular sieves for sorting molecules by size and shape e.g. for separating straight chain from branched alkanes when blending petrol.

Cracking hydrocarbons can also be carried out in the lab using an aluminium oxide catalyst. You should be able to draw the equipment set-up and describe the method.
If you haven’t done this practical, you can watch it here:
Isomerisation
Branched alkanes can also be made by isomerisation. In this process straight chain alkanes from the gasoline fraction are heated to 150°C in the presence of a platinum / aluminium oxide catalyst. The chains break apart and the fragments then rejoin forming branched chain molecules. The mixture is then passed over zeolite which acts as a molecular sieve, separating the branched from the straight chain products.

Catalytic reforming
Catalytic reforming is the process used to turn straight chain alkanes into cyclic alkanes (although it may also produce some branched alkanes, similar to isomerisation). Hydrocarbons from the naptha fraction are passed over a platinum / aluminium oxide catalyst at 500°C and a high pressure. Hydrogen is added to the mix to suppress the formation of carbon during the process which would deactivate the catalyst.

Practice questions
- Zeolites can be used as heterogeneous catalysts in the cracking of crude oil.
(a) Explain the terms heterogeneous and catalyst
(b) Write an equation, using molecular formulae, for the cracking of decane to produce and propene and another product.
(c) Zeolites can also be used as molecular sieves. Two structural isomers with the molecular formula C8H10 are shown below:
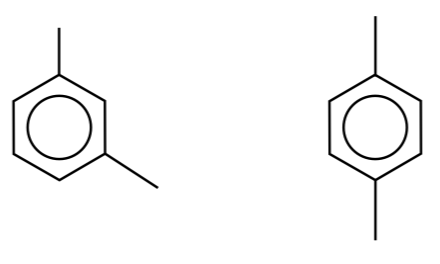
If a mixture of these isomers is passed through a zeolite sieve they can be separated. Suggest how this separation occurs with reference to zeolite structure and the shape of these isomers.
- Which of these statements are true and which are false?
A. A zeolite catalyst is used in the fractional distillation of crude oil
B. Heavy crude oil fractions are cracked to produce small chain alkanes and alkenes
C. Each fraction in the fractional distillation of crude oil contains a mixture of hydrocarbons
D. The high temperatures in the fractionating column are needed to break the covalent bonds in the molecules in order to separate them
E. Isomerisation is a process that produces cyclic molecules from straight chain molecules
F. A winter blend of petrol needs to be more volatile than a summer blend
3. The fluid catalytic cracker is designed to operate as a continuous process in which the powdered zeolite catalyst and the vaporised gas oil flow upwards through the riser reactor together. Explain how the properties of both the catalyst and the catalytic cracker are important industrially.
4. Write equations using molecular and skeletal formulae to show the reforming of heptane to form firstly methylcyclohexane and then methylbenzene. Explain why these reactions are not isomerisations.
Answers
- (a) Heterogeneous: a catalyst in a different physical state to the reactants
Catalyst: a substance that speeds up a reaction and can be recovered unchanged at the end / can be regenerated / lowers the activation enthalpy for the reaction
(b) C10H22 ⇾ C3H6 + C7H16
(c) A zeolite sieve has a honeycomb structure with pores / channels that are a similar size to that of the molecules being separated. 1,4-dimethylbenzene will be able to slip through the pores / channels but 1,3-dimethylbenzene will get stuck due to the position of its branches.
2. Statements B, C, F are true; A, D, E are false.
3. The zeolite catalyst has a very large surface area which facilitates the cracking of the long chain hydrocarbons in heavy gas oil into smaller alkanes and alkenes. It also allows for efficient energy transfer as the catalyst is heated and this heat energy is needed for the endothermic cracking reactions. The design of the catalytic cracker means the catalyst is easily removed from the reactor when spent and regenerated in a continuous process, and the energy is recycled. The products of the cracking reactions are gaseous and flow direct to a fractionating column to be separated into useful fractions for the blending of petrol and as a feedstock for industry.
4. The reforming reactions are not isomerisations because the reactant and product are not isomers – they have different molecular formulae.
